Simplifying clinical trial search & access
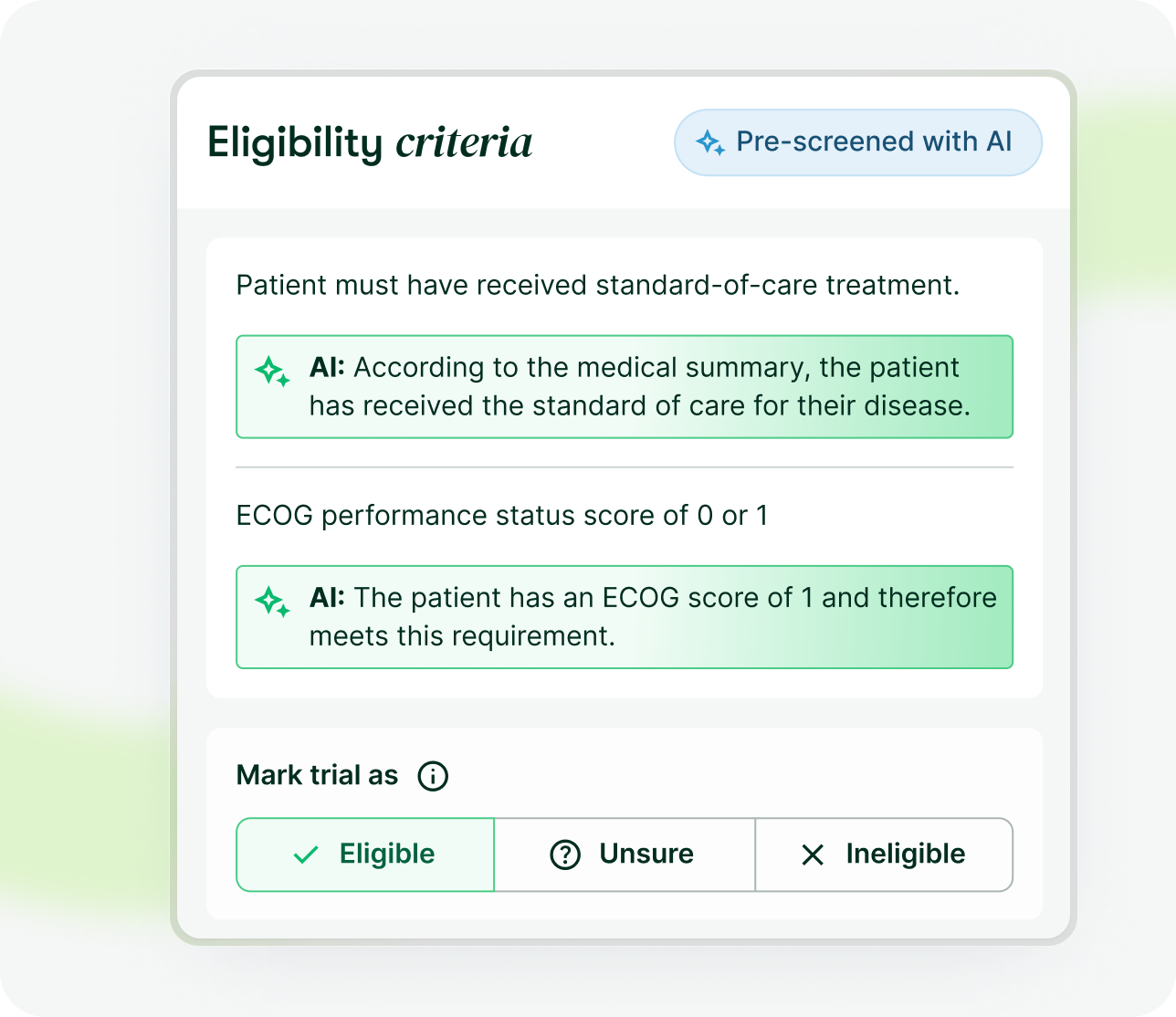
myTomorrows simplifies the process of identifying clinical trials, pre-screening patients against eligibility criteria, and communicating with recruiting trial sites.
We provide new options for your patient by helping you contact site staff and exchange medical information using a GDPR-compliant and ISO27001-certified platform. Our dedicated Medical Community team is also on hand to support you every step of the way.
When clinical trials are not an option, myTomorrows provides personalized support in acquiring treatment under an expanded access (compassionate use) program.
Our platform and services are free-of-charge to patients and physicians.